|
Pharmacogn Res. 2022; 14(1):53-60 A Multifaceted Journal in the field of Natural Products and Pharmacognosy www.phcogres.com | www.phcog.net |
Original Article |
GC-MS Analysis and Thrombolytic Property of Methanolic Leaf Extracts of Terminalia pallida Brandis against Carrageenan Instigated Tail Thrombosis Model in Mice
Sarvan Kumar Guguloth1,2, Narender Malothu1,*, Prasanth DSNBK1
Sarvan Kumar Guguloth1,2, Narender Malothu1,*, Prasanth DSNBK1
1Department of Pharmaceutical Chemistry, KL College of Pharmacy, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, Andhra Pradesh, INDIA.
2Department of Pharmacology, Vijaya College of Pharmacy, Munaganoor, Hayathnagar, Ranga Reddy, Telangana, INDIA.
Correspondence
Dr. M Narender
Assistant Professor, Department of Pharmaceutical Analysis, KL College of Pharmacy, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, Andhra Pradesh, INDIA.
Email id: narendermalothu@gmail.com
History
• Submission Date: 05-10-2021;
• Review completed: 28-10-2021;
• Accepted Date: 17-11-2021.
DOI : 10.5530/pres.14.1.9
Article Available online
http://www.phcogres.com
Copyright
© 2022 Phcog.Net. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license.
ABSTRACT
Background: Several plants of Terminalia species are being reported as medicinally useful. Terminalia pallida Brandis is one of the plants of the Combretaceae family, which constitutes several pharmacologically active substances. Present work explored leaf extract’s thrombolytic property, which could serve as the superior choice for currently using drugs. Objectives: The present study primarily focused on investigating the phyto-constituents such as phenolic and flavonoid compounds in methanolic leaf extract followed by GC-MS analysis and determining the in vitro thrombin inhibition and in vivo thrombolysis activity. Materials and Methods: Swiss albino mice (total = 36) were randomly distributed into six groups (n=6). Thrombosis was instigated by injecting 40 µL of 1% carrageenan (Type-I) via the subplantar region of the right hind paw of each mouse. The plant extract was screened for its in vitro thrombin inhibitory potency. In animal studies, the size of the blood clots in mouse tails was recorded by administering the plant extracts at 100, 200 and 300 mg/kg body weight for every 24, 48 and 72 hr, respectively. Results: A dose of 200 and 300 mg/kg of the extract showed significant thrombolytic activity at 24, 48, and 72 hr (p< 0.001) in a concentration-dependent manner when correlated with the control group. A reduction in the length of blood clots (p< 0.01) was observed at 48 and 72 hrs. In acute oral toxicity study, administration of leaf extract showed no mortality and no significant behavioral changes up to 2000 mg/kg dose. The GC-MS analysis explored the occurrence of about 16 eluted compounds; among these, few have been reported as medicinally useful, which accounts for the therapeutic importance of the plant. Conclusion: In conclusion, the methanolic extract of T. pallida Brandis leaves at 200, and 300 mg/kg showed significant inhibition of the Thrombosis in carrageenan instigated model of mice and in vitro thrombin activity.
Key words: Thrombosis, Carrageenan, Terminallia pallida Brandis, Heparin, Quercetin.
Cite this article: Guguloth SK, Malothu N, Prasanth DSNBK. GC-MS Analysis and Thrombolytic Property of Methanolic Leaf Extracts of Terminalia pallida Brandis against Carrageenan Instigated Tail Thrombosis Model in Mice. Pharmacog Res. 2022;14(1):53-60.

INTRODUCTION
Development of clot or Thrombosis is a tricky biochemical and physiological cascade participated by various physiological factors, including rupture of blood vessels, adhesion, and aggregation of platelets.[1,2] Thrombosis is one of the significant causes of morbidity and mortality throughout the world and is responsible for developing various cardiovascular and cerebral diseases with a high impact on the health and socio-economic status of the population. [3] Stroke and heart attacks are mainly triggered by forming a clot in the arteries and atherosclerotic plaques.[4] The haemopoietic system plays a significant role in homeostasis between forming a clot (fibrin) and its lysis (fibrinolysis) to prevent and protect from blood loss and ensure tissue perfusion by forming a platelet plug.[5] To protect against hemorrhage, the physiological interaction between clotting factors and platelets occurs, followed by formation of a hemostatic plug that could stop the free flow of blood at the site of vascular injury. The principal enzyme responsible for the clotting process is thrombin, while plasmin is an enzyme responsible for fibrin and fibrinogen degradation.[6,7] Several synthetic and semi-synthetic drugs such as oral anticoagulants, antiplatelets, or thrombolytics are available for treating various thrombotic disorders (vascular blockage, myocardial or cerebral infarction, venous thromboembolism, and deep vein thrombosis). Most of these drugs exhibit adverse effects like bleeding, severe anaphylactic reactions, overarching safety, and efficacy.[8] Approximately 4% of the world’s population has been experiencing excessive bleeding or hemorrhage, 14% of patients worldwide require a blood transfusion. These findings suggest that bleeding is the most alarming side effect of modern synthetic medicines evokes cardiovascular outcomes and carries the risk of death.[9,10]
Despite in cardiovascular diseases the development of coagulopathy is also one of the important signs of poor prognosis in patients affected by recent pandemic Covid-19. Elevation of marked D-dimer is a predictor for mortality and hospitalization of Covid-19 patients. The severity of COVID-19 is being associated with coagulation, use of anticoagulants potentially improve prognosis according to various reports from a retrospective study.[11] In the connection of development of new effective anticoagulants the plant extracts have been found as most effective and preventable alternative medicines for thrombus-related diseases.[12] As per the World Health Organization (WHO) reports majority of the populations (over 80%) in developing countries are being using herbal drugs in their health care.[13] As per the recent reports the variety of phytochemical constituents such as coumarins, flavonoids, tannins, and phenols were isolated and identified their significance in the hemostasis.[14] A naturally occurring plant agent rutin, also called rutoside, is chemically flavonoid glycoside abundantly present in Fagopyrum esculentum (buckwheat), showed effective thrombolytic activity in mice and humans (IC value is 6.1 µM). The plant constituent rutin has been proved that it is reversibly inhibiting 50 the protein disulfide isomerase in the process of anticoagulation.[15] In addition, various potential plant extracts and constituents like borneol,[16] sulfated (1-3)-β-L-arabinan of Codium vermilara,[17] crude extract of Erigeron canadensis L,[18] 2,3,5,4-tetrahydroxy stilbene-2-Oβ-D-glucoside of Polygonum multiforum,[19] salvianolic acid B-Salvia miltiorrhiza,[20] pomolic acid-Licania pittier,[21] rhynchophylline,[22] with probable mechanism of action have been reported in various literature sources. Though, no new herbal agent has been established for complete anticoagulant therapy. Hence, there is an urge for the development and exploration of potential medicine to treat Thrombosis with safe and effective therapy with no side effects.
Terminalia pallida Brandis is a plant of the Combretaceae family, commonly found in tropical and subtropical countries [Figure 1]. It is called with different local names in the southern region of India (i.e., Tellakaraka and Velmakaraka in Telugu, Vallaikkadukkay in Tamil, and white gallnut tree in English.[23]

Figure 1: Plant material of T. pallida Brandis a) Plant leaf b) Leaf powder.
The plant part constitutes plenty of medicinally useful phytoconstituents. Fruits possess anti-diabetic properties,[24] and the leaves are being used in various herbal formulations, pharmaceuticals, and animal husbandry.[25] The powdered form of fruit concoction is being used to treat the hemorrhoids.[26] In the connection of our investigations on the assessment of thrombolytic properties of T. pallida Brandis,[27] the leaf extract was tested to know the thrombin inhibition property as the enzyme is crucial in clot formation. Further, it’s in vivo thrombolytic activity was determined on animal studies using carrageenan instigated tail thrombosis model of mice. The carrageenan induced tail thrombosis model chosen as it is non-invasive type and simple to perform on small laboratory animals with stress free conditions.[28,29] The present communication described the findings of phytochemical investigation, GC-MS analysis and in vivo thrombolytic activity of METP.
MATERIALS AND METHODS
Chemicals
Carrageenan type-I, low molecular weight heparin, methanol, DMSO, quercetin etc., utilized in this work were procured from SD Fine Chemicals, Mumbai, India.
Collection and authentication of plant
As this plant is widely distributed in the southern region of India, the leaves were collected from Tirumala hills, Chittoor, Andhra Pradesh and authentication of the plant was done by Dr. K. Madhava Shetty, Taxonomist, Sri Venkateswara University, Tirupati, and Andhra Pradesh. A copy of the sample (0821) was referenced for the future.
Procedure for extraction
The leaves were desiccated at ambient temperature (in the air). Dried leaves were powdered and weighed about 250 gm for solvent extraction. The powder was macerated for 24-72 hr and subsequently extracted with methanol (1 lit). Resulted leaf extract was concentrated using a rotary evaporator (in vacuo) and was preserved in cold conditions.[27,30]
Estimation of total flavonoid content
Flavonoids are the clusters of polyphenolic admixture, which manifest diverse pharmacological outcomes. It was performed by treating about 2.5 mL of leaf extract with AlCl3 (2.5 mL) in 90% ethanol and kept at ambient temperature for 40 min. [31] The absorbance of resulted mixture was recorded at 415 nm by using a UV-visible spectrophotometer (Shimadzu 1501 model). The experiments were conducted in triplicate by taking ethanol as a blank and quercetin as a reference standard. The total flavonoid content was estimated and indicated in µg/QE/mg of dry extract.
Estimation of total phenolic content
Secondary metabolites of plants consist of the rich source of phenols which usually possess a wide range of pharmacological properties. The test was performed with FC reagent using pyrocatechol as the standard.[31,32] The plant extract (about 0.2 mL) was treated with 10 % w/v of FC (1 mL) reagent in 7.5 % w/v sodium carbonate (0.8 mL). Resulted mixture was kept in incubation for 1 hr and the phenolic content was calculated based on absorbance recorded at 760 nm. The total phenolic content indicated in mg/g of gallic acid equivalents in milligrams per gram (mg GAE/g) of dry extract.
Experimental animals
The Swiss albino mice weighing between 30-35 grams were employed in the present work. The experimental mice were procured from registered breeders (Venkateswara enterprises, Hyderabad) and were maintained under standard conditions (temperature-22 ±2°C; relative humidity-30-70 %) with a 12:12 light-dark cycle. Also, the animals were fed with a standard pellet diet and water ad libitum. The IAEC was approved (1292/ac/09/CPCSEA/47/A) the proposed experiment protocols at Vijaya College of Pharmacy, affiliated to JNTUH, Munaganoor, Hayathnagar, and Ranga Reddy.
Thrombin inhibition assay
Thrombin inhibition assay was performed by employing standard protocol’s described by Batra et al.[33] Initially, the METP (as thrombin inhibitor) was incubated with Tris-buffer at pH 7.5 in a 96 well plate. Then, thrombin substrate III (0.2 mM, prepared from Kit) followed by thrombin (1U/mL) was added to incubate. After incubation for 1 hr the 96-well plate was read for fluorescence intensity by taking 450 nm as emission wavelength and 390 nm as excitation wavelength on fluorimeter (Spectra Max M5e, Molecular Devices). The reduction in flouroscence intensity to that of concentration of METP was measured and calculated the percentage (%) inhibition of thrombin activity.
Experimental study design
Swiss albino mice (Total-36 nos) were randomly divided into 6 groups (n=6). The leaf extracts were disintegrated in 20% v/v DMSO in normal saline (diluent). The control group was injected with DMSO (20% v/v) and the experimental treated groups were injected through the intraperitoneal route with 100, 200, and 300 mg/kg of METPs. The standard group animals were injected with 10 IU and 100 IU of low molecular weight heparin. To instigate the blood clot in the tail the animals were injected with 40 µL of 1% carrageenan (type-I) in the subplantar region of the right hind paw of each mouse after one hour of every dose of leaf extract.[34] The size (mm) of blood clots in the tail was recorded every 24, 48, and 72 hrs after the injections of carrageenan, respectively.
Determination of acute oral toxicity (LD50): Acute oral toxicity studies of METP of T. pallida Brandis performed according to OECD guideline 425.[35] A total of 28 Swiss albino mice (25-30 gm) in seven groups were employed for toxicity studies. Group I served as control and to the other experimental groups 50, 100, 200, 400, 600 and 2000 mg/kg of the METP was administered using oral feed, respectively. All the mice were noticed for general behavioral changes; signs of toxicity and mortality after treatment for the first 6, 14, and 24 hr, after that daily for 14 days.
GC-MS analysis: GC-MS is one the effective chemical analytical method for confirming phytoconstituents. It will provide an emissary spectral output of all the probable components that get separated from the sample. The procedure involves by injection of the sample to the port of the GC-MS device where vaporization takes place followed by separation was achieved with an analyzer. Each component was producing an ideal specific peak, which is recorded on a paper chart electronically. GC-MS analysis of METP was performed at CSIR-Indian Institute of Chemical Technology (IICT), Habsiguda, Hyderabad. The data was recorded on combined gas chromatogram system (Agilent GC-MS5977B) and mass spectrometer, fitted with an HP-5 MS fused silica column (5% phenyl methyl siloxane 30.0 m × 250 μM, film thickness 0.25 μM), interfaced with 5675C Inert MSD with Triple-Axis detector.[36]
Statistical Analysis
The results of the entire study were statistically presented by using software (Graph pad prism, Version 5.0). The statistical divergence was explored by one-way analysis of variance by Tukey’s multiple comparison tests. The obtained data were notified as mean ± standard error mean and the level of significance was contemplated as p ≤0.05.
RESULTS
Total phenolic and flavonoid content in METP
The macerated solvent extract of leaves was concentrated which gave about 2.3 % w/w crude methanolic extract. In which the total phenolic content was found as 16.97 mg GAE/g. While the total flavonoid content in obtained was 7.42 mg/QE/mg.
In vitro thrombin inhibition activity
The crude METP was incubated in buffer solution with thrombin substrate (50 µg/mL-2 mg/mL) for 5 min, then with thrombin (1U/mL). The fluorescence was read out was recorded by fluorescence reader after 60 min. Treatment of methanolic leaf extract showed thrombin inhibition in dose dependent manner. The maximum thrombin inhibition (93.76±2.98%) was achieved at 2 mg/mL. Moderate to remarkable inhibition was observed for test concentration 50-200 µg/mL. Moreover, there was substantial increment in percent inhibition was found for 500 µg/mL, 800 µg/mL and 1 mg/mL of METP (Table 1, Figure 2).
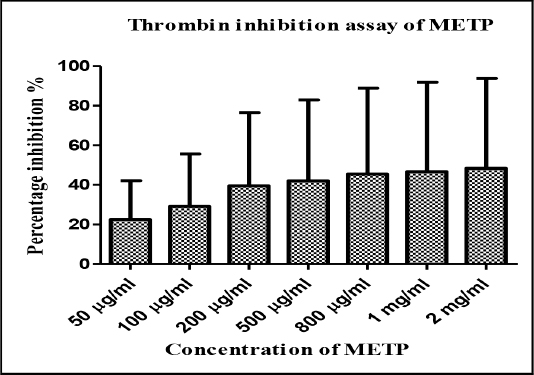
Figure 2: Graphical report of thrombin inhibitory activity of METP.
Table 1: Thrombin inhibitory activity data of METP.
| S. No | Concentration of METP | Percentage inhibition (% Mean±SEM) |
|---|---|---|
| 1 | 50 µg/mL | 42.10±2.75 |
| 2 | 100 µg/mL | 55.64±2.60 |
| 3 | 200 µg/mL | 76.46±2.65 |
| 4 | 500 µg/mL | 82.85±1.18 |
| 5 | 800 µg/mL | 88.90±2.10 |
| 6 | 1 mg/mL | 91.85±1.58 |
| 7 | 2 mg/mL | 93.76±2.98 |
Note: SEM-Standard Error Mean.
In vivo thrombolytic activity
Test groups-III and IV treated with plant extract (at 200 and 300 mg/kg) were showed significant activity at 24 hr (p< 0.001) to that of control group [Figure 3]. Also it showed a reduction in length of blood clots (p< 0.01) at 48 and 72 hr at 200 and 300 mg/kg. The standard group (Group-V) treated with 10IU of low molecular weight heparin was not shown significance at 24, 48 and 72 hr when correlated with the control group [Figure 4]. The thrombolytic activity of METPs against carrageenan instigated tail thrombosis model in mice at 24, 48 and 72 hr was summarized in Table 2.

Figure 3: Thrombolytic activity of Group-I (Normal Control). Group-II-IV (METP of T. pallida Brandis at 100, 200 and 300 mg/kg), and Group-V and VI (Standard Heparin) on carrageenan instigated tail thrombosis model in mice.

Figure 4: Photographs of tail thrombosis of mice tail at 48 hr, showing thrombolytic effect of Normal Control (Group I), METP of T. pallida Brandis (Group-II-IV) and standard (Group-V and VI) in carrageenan instigated thrombosis mice model (after 48 hr carrageenan injection).
Table 2: Thrombolytic activity of METP in carrageenan instigated tail thrombosis model in mice.
| Experimental group | Dose | Inhibition of blood clot in the tail (%Mean±SEM, n=6) | ||
|---|---|---|---|---|
| 24 hr | 48 hr | 72 hr | ||
| I | 20% DMSO | 13.63±1.21 | 15.88±0.38 | 11.04±1.63 |
| II | 100 mg/kg | 28.43±1.52 | 32.43±2.12 | 54.73±1.58 |
| III | 200 mg/kg | 56.30±1.57* | 60.46±1.15** | 64.89±2.01** |
| IV | 300 mg/kg | 66.68±2.32** | 74.45±1.02** | 77.65±1.09*** |
| V | 10 IU | 15.89±0.99 | 11.08±0.65 | 14.36±0.69 |
| VI | 100 IU | 68.79±2.61** | 72.62±1.08*** | 78.31±2.27*** |
Note: Group I = Normal control; Group II, III and IV = METP of T. pallid brandis (100, 200 and 300 mg/kg) Group V and VI = Low molecular weight heparin; Data were represented as Mean±SEM (n=6).*p<0.05, **p<0.01, ***p<0.001 significant when correlated control group.
Acute toxicity profile
In oral toxicity study, administrations of 50, 100, 200, 400, 600 and 2000 mg/kg of METP were not produced any clinical sign of toxicity, as well as no animal was died. The behavioral patterns of animals were recorded for the first 6, 14, and 24 hr after the administration of plant extract. The test animals were found normal and did not showed any notable changes in behavior, skin reactions. Further no animals were noticed the disability in food and water intake, breathing as well as postural abnormalities and signs of alopecia.
GC-MS analysis of METP
In the GC-MS chromatogram of METP, a total number of 16 compounds were identified [Figure 5]. The compounds were eluted with different retention times and peak area which designates the presence of chemically diverse plant constituents. The retention time (Rt), peak area (A) and percentage (A %) of the eluted components were depicted in Table 3.
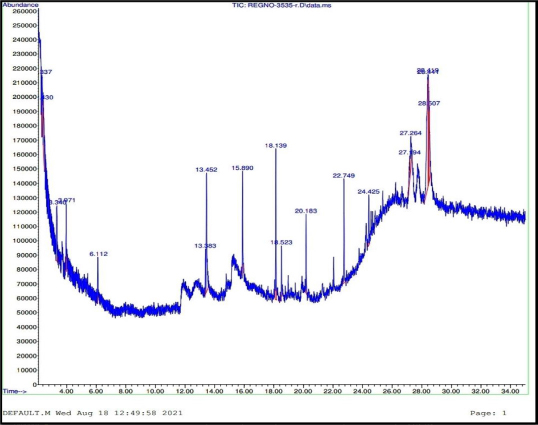
Figure 5: GC-MS chromatogram of METP of T. pallida Brandis.
Table 3: Chromatographic parameters of GC-MS report.
| S. No. | Chemical Name | Retention time (min) | Peak area | Percentage (%) |
|---|---|---|---|---|
| 1 | N,N-Dimethylformamide dipropyl acetal | 2.33 | 1014006 | 3.98 |
| 2 | Propyl carbamate | 2.43 | 1043856 | 4.10 |
| 3 | Methyl carbamate | 3.34 | 1245544 | 4.88 |
| 4 | 1,2,4 Butanetriol | 3.97 | 862219 | 3.38 |
| 5 | Ethyl(dimethyl)isopropoxysilane | 6.11 | 502932 | 1.97 |
| 6 | 3,7-Ditert-butylnaphthalen-1-ol | 13.45 | 2643455 | 10.37 |
| 7 | Octadecane | 18.13 | 1839919 | 7.22 |
| 8 | Adrenaline | 18.52 | 518930 | 2.04 |
| 9 | Eicosane | 20.18 | 879341 | 3.45 |
| 10 | Benzo[b]thiophen-2-amine, 3-phenyl-N-(phenylmethylene) | 22.74 | 1420282 | 5.57 |
| 11 | Octadecanoic acid, tert-butyldimethylsilyl ester | 24.42 | 786513 | 3.09 |
| 12 | Decamethyltetrasiloxane | 27.19 | 585772 | 2.30 |
| 13 | Benzo[h]quinoline, 2,4-dimethyl | 27.26 | 951148 | 3.73 |
| 14 | Benzoic acid, 2,4-dimethyl-, (2,4-dimethylphenyl) methyl ester | 28.41 | 5321081 | 20.88 |
| 15 | Pyridine, 3-[2,2-bis(trimethylsilyloxy) vinyl]- | 28.43 | 951148 | 10.25 |
| 16 | Cyclotrisilioxane hexamethyl | 28.50 | 5321081 | 6.29 |
In GC-MS analysis, the phyto-constituents were characterized by considering the percent elution followed by interpretation of mass spectrum with appropriate hits (NIST library etc). The molecular weight (MW), molecular formula (MF) and structure of characterized compounds are summarized in Table 4. Majority of the identified compounds were found biological active and they are reported as anticonvulsant, muscle relaxant (Propyl carbamate),[37] anti-cancer, anti-diabetic (Cyclotrisilioxane hexamethyl),[38] estrogenic (Decamethyltetrasiloxane),[39] and anti-oxidant (Adrenaline),[40] agents [Table 4].
Analysis of chromatogram and mass spectrum data of hit compounds revealed the presence of variety of plant components. Among these pyridine, 3-[2, 2-bis (trimethylsilyloxy) vinyl] - (20.88%), 3,7-ditertbutylnaphthalen-1-ol (10.252 %) were comprised majorly. The mass spectrums of the eluted compounds were shown in Figure 6.
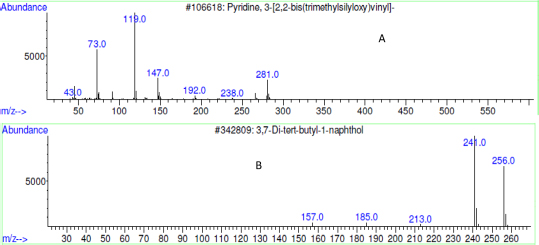
Figure 6: Mass spectrum of eluted compounds pyridine, A. 3-[2,2-bis(trimethylsilyloxy)vinyl]- (20.88%); B. 3,7-Ditert-butylnaphthalen-1-ol (10.252 %).
Table 4: Chemical composition identified in METP extract by GC-MS.
| S. No. | Chemical Name | IUPAC Name | Mass (g/mol) | Molecular formula | Structure |
|---|---|---|---|---|---|
| 1 | N,N-Dimethylforma-mide dipropyl acetal | N,N-Dimethyl-1,1-dipropoxymethanamine | 175.27 | C9H21NO2 | 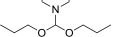 |
| 2 | Propyl carbamate | Propyl carbamate | 103.12 | C4H9NO2 | 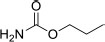 |
| 3 | Methyl carbamate | Methyl carbamate | 75.03 | C2H5NO2 | 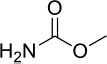 |
| 4 | 1,2,4 Butanetriol | 1,2,4-Butanetriol |  |
||
| 5 | Ethyl(dimethyl)isopropoxysilane | Ethyl(dimethyl)isopropoxysilane | 146.11 | C7H18OSi | 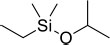 |
| 6 | 3,7-Ditert-butyl naphthalen-1-ol | 3,7-Ditert-butyl naphthalen-1-ol | 256.34 | C18H24O |  |
| 7 | Octadecane | Octadecane | 254.49 | C18H38 |  |
| 8 | Adrenaline | 4-[1-Hydroxy-2-(methyl amino) ethyl] benzene-1,2-diol | 183.2 | C9H13NO3 | 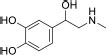 |
| 9 | Eicosane | Eicosane | 282.33 | C20H42 |  |
| 10 | Benzo[b]thiophen-2-amine, 3-phenyl-N-(phenylmethylene) | 1-Phenyl-N-(3-phenyl-1-benzo thiophen-2-yl) methanimine | 313.4 | C21H15NS | 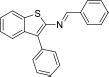 |
| 11 | Octadecanoic acid, tert-butyldimethylsilyl ester | t-Butyl(dimethyl) silyl stearate | 188.12 | C9H20O2Si | 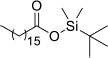 |
| 12 | Decamethyltetrasiloxane | [Dimethyl(trimethylsilyloxy)silyl]oxy-dimethyl-trimethylsilyloxysilane | 310.68 | C10H30O3Si4 |  |
| 13 | Benzo[h]quinoline, 2,4-dimethyl | 2,4-Dimethylbenzo[h] quinoline | 207.10 | C15H13N | 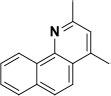 |
| 14 | Benzoic Acid, 2,4-Dimethyl-, (2,4-Dimethylphenyl) Methyl Ester | (2,4-dimethylphenyl)methyl 2,4-dimethylbenzoate | 268.3l | C18H20O2 | 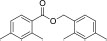 |
| 15 | Pyridine, 3-[2,2-bis(trimethylsilyloxy)vinyl]- | Trimethyl-(2-pyridin-3-yl-1-trimethylsilyl oxyethenoxy)silane | 281.50 | C13H23NO2Si2 |  |
| 16 | Cyclotrisilioxane hexamethyl | 2,2,4,4,6,6-Hexamethyl-1,3,5,2,4,6-trioxatri silinane | 222.46 | C6H18O3Si3 | 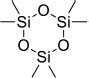 |
DISCUSSION
Polyphenols and flavonoids are secondary plant metabolites naturally present in plants. As per earlier reports, most flavonoids possess antithrombotic properties.[41-44] the preliminary phyotchemical study confirmed the occurrence of both flavonoid and phenolic compounds in methanolic extract. The in vitro thrombolytic screening (clot lysis: 95.43%±0.697) results showed potent activity at the test concentration (800 µg/mL) in our earlier studies.[27] Furthermore, the plant extract was tested with thrombin inhibition assay. In these studies, METP showed inhibition of thrombin activity (93.76±2.98%) at 2 mg/mL. The thrombin inhibition property indicated us to screen the effect of plant extract in Thrombosis induced animal model. In this connection the thrombolytic property of leaf extract was assessed in animal studies. The formation of a blood clot in vascular-related diseases seems to be one of the important risk factors which are responsible for mortality and morbidity in the world.[45] By considering the suitability of the study the carrageenan-instigated tail thrombosis model was employed to know the thrombolytic behavior. In the carrageenan-induced tail thrombosis model, a red color appears in the tip of the tail of the experimental animal and the length of the blood clot in the tail (thrombosis) increased with the time transpired followed by development of necrosis.[34] Results obtained suggested that in vivo thrombolytic effect of METP at 200 and 300 mg/kg at 24, 48 and 72 hr (p<0.001) significant thrombolytic activity in concentration dependent manner. In general, oral acute toxicity studies are being conducted to acquire suitable dose for chronic toxicity tests and find out the pretentious organs at the end of the treatment.[46,47] In oral acute toxicity, at the preferred higher dose (2000 mg/kg) the plant extract was not showed any significant toxicological signs, and mortality throughout the 14 days of treatment.
The preliminary GC-MS investigations confirmed the constitution of phenolic compounds in the plant extracts, which was correlating with the phytochemical tests. Probably, occurrence of these constituents primarily indicated the thrombolytic properties of plant leaves. Further studies such as isolation and structure elucidation of the components of the methanolic extract could explore the active principle there by possible mechanism of action.
CONCLUSION
In the present study, the preliminary phytochemical investigations were indicated the presence of phenolic and polyphenolic compounds in the methanolic extracts. In thrombin inhibition assay, the plant extract showed 93.76±2.98% of inhibition at 2 mg/mL which indicated its thrombin lysis property. In the assessment of thrombolytic property against carrageenan instigated tail thrombosis in mice model showed significant inhibition (clot lysis-67.45±1.02**) of Thrombosis at 200 and 300 mg/kg of plant extract in concentration dependent manner. However, the exact mechanism of the blood clot dissolving property of these extracts was needed to be established. The GC-MS studies confirmed the presence of biologically active constituents. As per the analysis the methanolic extract was majorly comprised with phenolic compounds. This study could serve as a new insight for future investigation for herbal thrombolytic agent’s which can lead the identification and characterization.
ACKNOWLEDGEMENT
The authors are thankful to Vijaya College of Pharmacy, Munaganoor, Hyathnagar, Ranga Reddy and KL College of Pharmacy, KL University, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, Andhra Pradesh for providing laboratory facilities to carry out this research study. Authors are thankful to the Scientist in-charge, ASRC, CSIR-IICT, Habsiguda, and Hyderabad for providing GC-MS analysis reports.
CONFLICT OF INTEREST
The authors declare that there is no conflict of statement.
ABBREVIATIONS
GC-MS: Gas Chromatography-Mass Spectrometry; OECD: Organization for Economic and Cooperation Development; IAEC: Institutional Animal Ethical Committee; METP: Methanolic extract of Terminalia pallida Brandis; FC: Folin Ciocalteu’s; T. pallida: Terminalia pallida Brandis; DMSO: Dimethyl Sulfoxide.
REFERENCES
1. Hsiao G, Yen MH, Lee YM, Sheu JR. Antithrombotic effect of PMC, a potent alpha-tocopherol analogue on platelet plug formation in vivo. Br J Haematol. 2002;117(3):699-704. doi: 10.1046/j.1365-2141.2002.03492.x, PMID 12028044.
2. Jørgensen L. The role of platelets in the initial stages of atherosclerosis. J Thromb Haemost. 2006;4(7):1443-9. doi: 10.1111/j.1538-7836.2006.02006.x, PMID 16839335.
3. Froemel D, Fitzsimons SJ, Frank J, Sauerbier M, Meurer A, Barker JH. A review of thrombosis and antithrombotic therapy in microvascular surgery. Eur Surg Res. 2013;50(1):32-43. doi: 10.1159/000347182, PMID 23548333.
4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: A major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147(9):1561-4, PMID 3632164.
5. Riddel JP, Aouizerat BE, Miaskowski C, Lillicrap DP. Theories of blood coagulation. J Pediatr Oncol Nurs. 2007;24(3):123-31. doi: 10.1177/1043454206298693, PMID 17475978.
6. Pereira B, Brazón J. Aqueous extract from Brownea grandiceps flowers with effect on coagulation and fibrinolytic system. J Ethnopharmacol. 2015;160:6-13. doi: 10.1016/j.jep.2014.11.022, PMID 25460592.
7. Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55(11):1409-15. doi: 10.1001/ archneur.55.11.1409, PMID 9823823.
8. Rajput MS, Mathur V, Agrawal P, Chandrawanshi HK, Pilaniya U. Fibrinolytic activity of kaempferol isolated from the fruits of Lagenaria siceraria (Molina) Standley. Nat Prod Res. 2011;25(19):1870-5. doi: 10.1080/14786419.2010.540760, PMID 21861768.
9. Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24(20):1815-23. doi: 10.1016/s0195-668x(03)00485-8, PMID 14563340.
10. Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators, Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354(14):1464-76. doi: 10.1056/NEJMoa055443, PMID 16537663.
11. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-9. doi: 10.1111/jth.14817, PMID 32220112.
12. Tsasi G, Mailis T, Daskalaki A, Sakadani E, Razis P, Samaras Y, et al. The effect of harvesting on the composition of essential oils from five varieties of Ocimum basilicum L. cultivated in the island of Kefalonia, Greece. Plants (Basel). 2017;6(3):41. doi: 10.3390/plants6030041, PMID 28927018.
13. Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. Evid Based Complement Alternat Med. 2007;4(Suppl 1);Suppl 1:37-40. doi: 10.1093/ ecam/nem096, PMID 18227931.
14. Van Doormaal FFV, Büller HR, Middeldorp S. Development in anticoagulant therapy. Crit Rev Oncol Hematol. 2008;66(2):145-54. doi: 10.1016/j. critrevonc.2007.09.009, PMID 18032061.
15. Dar MA, Tabassum N. Rutin-potential natural thrombolytic agent. Int Curr Pharm J. 2012;1(12):431-35.
16. Li YH, Sun XP, Zhang YQ, Wang NS. The antithrombotic effect of borneol related to its anticoagulant property. Am J Chin Med. 2008;36(4):719-27. doi: 10.1142/ S0192415X08006181, PMID 18711769.
17. Fernández PV, Quintana I, Cerezo AS, Caramelo JJ, Pol-Fachin L, Verli H, et al. Anticoagulant activity of a unique sulfated pyranosic (1->3)-β-L-arabinan through direct interaction with thrombin. J Biol Chem. 2013;288(1):223-33. doi: 10.1074/ jbc.M112.386441, PMID 23161548.
18. Pawlaczyk I, Czerchawski L, Kuliczkowski W, Karolko B, Pilecki W, Witkiewicz W, et al. Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thromb Res. 2011;127(4):328-40. doi: 10.1016/j. thromres.2010.11.031, PMID 21172723.
19. Xiang K, Liu G, Zhou YJ. 2, 3, 5. 4′-tetrahydroxy stilbene-2-O--D-glucoside (THSG) attenuates human platelet aggregation, secretion and spreading in vitro. Thromb Res. 2014;133(2):211-17.
20. Ma C, Yao Y, Yu QX, Zhou WX, Yang PY, Wu WY, et al. Differential proteomic analysis of platelets suggested possible signal cascades network in platelet streated with salvianolic acid B. PLOS ONE. 2011;6(2):e46921.
21. Alvarado-Castillo CA, Estrada O, Carvajal E. Pomolic acid, triterpenoid isolated from Licania pittieri, as competitive antagonist of ADP-induced aggregation of human platelets. Phytomedicine. 2012;19(6):484-87. doi: 10.1016/j.phymed. 2011.12.011, PMID 22402243.
22. Xie XL, Gong Q, Lueta Y, Deng J, Nie J, Chen WM, et al. Effect of rhynchophylline on platelet aggregation and cytoplasmic free calcium level in rabbits. Chin J Pharmacol Toxicol. 2011;25:68-71.
23. Latheef SA, Prasad B, Bavaji M, Subramanyam G. A data on endemic plants at Tirumala hills in India. Bio-Information. 2008;2(6):260-2.
24. Kameswara Rao B, Renuka Sudarshan P, Rajasekhar MD, Nagaraju N, Appa Rao Ch. Antidiabetic activity of Terminalia pallida fruit in alloxan induced diabetic rats. J Ethnopharmacol. 2003;85(1):169-72. doi: 10.1016/s0378-8741(02)00396-3, PMID 12576217.
25. Raju SAJ, Lakshmi PV, Ramana KV. Reproductive ecology of Terminalia pallida Brandis (Combretaceae), an endemic and medicinal tree species of India. Curr Sci. 2012;102(6):909-17.
26. Fahmy NM, Al-Sayed E, Singab AN. Genus Terminalia: A phytochemical and biological review. Med Aromat Plants. 2015;4(5):2-21.
27. Guguloth SK, Malothu N, Kulandaivelu U, Rao KG, Areti AR, Noothi S. Phytochemical investigation and in vitro thrombolytic activity of Terminalia pallida Brandis leaves. Res J Pharm Technol. 2021;14(2):879-82. doi: 10.5958/0974-360X.2021.00156.6.
28. Kod’ousek R, Jezdínský J, Krajcí D. Histological and ultrastructural changes of cardiomyocytes in experimental rats with tail thrombosis following subplantar application of carrageenin. Med Princ Pract. 2007;16(5):360-6. doi: 10.1159/000104809, PMID 17709924.
29. Bekemeier H, Gabor M, Hirschelmann R. Influence of flavonoids and lanthanides on kappa-carrageenin rat tail thrombosis. Exp Pathol. 1990;40(1):61-3. doi: 10.1016/s0232-1513(11)80288-4, PMID 2279536.
30. Bembde NS, Meshram PV, Patil MK, Shaikh J. The preliminary phytochemical analysis of ethanolic extract of Tylophora indica. Res J Pharmacogn Phytochem. 2020;12(1):1-6. doi: 10.5958/0975-4385.2020.00001.1.
31. Hsu CY. Antioxidant activity of extract from Polygonum aviculare L Biol Res. 2006;39(2):281-8. doi: 10.4067/s0716-97602006000200010, PMID 16874403.
32. Gülçin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004;90(2-3):205-15. doi: 10.1016/j.jep.2003.09.028, PMID 15013182.
33. Batra S, Roy AK, Patra A, Bhaduri AP, Surin WR, Raghavan SAV, et al. Baylis-Hillman reaction assisted parallel synthesis of 3,5-disubstituted isoxazoles and their in vivo bioevaluation as antithrombotic agents. Bioorg Med Chem. 2004;12(9):2059-77. doi: 10.1016/j.bmc.2004.02.023, PMID 15080910.
34. Yan F, Yan J, Sun W, Yao L, Wang J, Qi Y, et al. Thrombolytic effect of subtilisin QK on carrageenan induced thrombosis model in mice. J Thromb Thrombolysis. 2009;28(4):444-8. doi: 10.1007/s11239-009-0333-3, PMID 19377880.
35. OECD guidelines for testing of chemicals, Test No. 425 TG. Acute toxic class method. Organization for Economic Cooperation and Development; 2008.
36. Olivia NU, Goodness UC, Obinna OM. Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of Hibiscus asper leaves. Futur J Pharm Sci. 2021;7(1):592-5. doi: 10.1186/s43094-021-00208-4.
37. Slater HR, Mckinney L, Shepherd J, Packard CJ. Receptor-independent low-density lipoprotein catabolism. Evaluation of 2-hydroxyacetaldehyde-treated lipoprotein as a probe for its measurement. Biochim Biophys Acta. 1984;792(3):318-23. doi: 10.1016/0005-2760(84)90199-1, PMID 6320900.
38. Matoševic A, Bosak A. Carbamate group as structural motif in drugs: A review of carbamate derivatives used as therapeutic agents. Arh Hig Rada Toksikol. 2020;71(4):285-99. doi: 10.2478/aiht-2020-71-3466, PMID 33410773.
39. Rutter S, Macduff C, Stones C, Gomez-Escalada M. Evaluating children’s hand washing in schools: An integrative review of indicative measures and measurement tools. Int J Environ Health Res. 2021;31(1):1-19. doi: 10.1080/09603123.2019.1625032. PMID 31204496.
40. He B, Rhodes-Brower S, Miller MR, Munson AE, Germolec DR, Walker VR, et al. Octamethylcyclotetrasiloxane exhibits estrogenic activity in mice via ERalpha. Toxicol Appl Pharmacol. 2003;192(3):254-61. doi: 10.1016/s0041-008x(03)00282-5, PMID 14575643.
41. Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic Biol Med. 1997;22(5):749-60. doi: 10.1016/s0891-5849(96)00351-6, PMID 9119242.
42. Kang WS, Lim IH, Yuk DY, Chung KH, Park JB, Yoo HS, et al. Antithrombotic activities of green tea catechins and (-)-epigallocatechin gallate. Thromb Res. 1999;96(3):229-37. doi: 10.1016/s0049-3848(99)00104-8, PMID 10588466.
43. Singh I, Mok M, Christensen AM, Turner AH, Hawley JA. The effects of polyphenols in olive leaves on platelet function. Nutr Metab Cardiovasc Dis. 2008;18(2):127-32. doi: 10.1016/j.numecd.2006.09.001, PMID 17346951.
44. Léger CL, Carbonneau MA, Michel F, Mas E, Monnier L, Cristol JP, et al. A thromboxane effect of a hydroxytyrosol-rich olive oil wastewater extract in patients with uncomplicated type I diabetes. Eur J Clin Nutr. 2005;59(5):727-30. doi: 10.1038/sj.ejcn.1602133, PMID 15798774.
45. Yang SA, Im NK, Ji YJ, Yoo DC, Jhee KH, Lee IS. Radical scavenging and inhibition of platelet function by a polyphenol-rich fraction from Salvia miltiorrhiza Bunge. TONPJ. 2008;1(1):7-13. doi: 10.2174/1874848100801010007.
46. Schussheim AE, Fuster V. Thrombosis, antithrombotic agents, and the antithrombotic approach in cardiac disease. Prog Cardiovasc Dis. 1997;40(3):205-38. doi: 10.1016/s0033-0620(97)80035-7, PMID 9406677.
47. Sasidharan S, Darah I, Jain K. In vivo. and in vitro. Toxicity Study of Gracilaria changii. Pharm Biol. 2008;46(6):413-17. doi: 10.1080/13880200802055867.
GRAPHICAL ABSTRACT
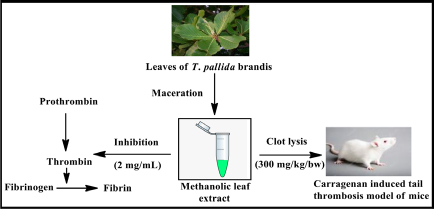
SUMMARY
The methanolic extract obtained from the dried leaves of T. Pallida Brandis was screened for flavonoid and phenolic content and evaluated the GC-MS data. In vitro thrombin inhibition assay was tested for extracts. Further the thrombolytic activity was tested on carrageenan instigated tail thrombosis animal model in mice. In these studies GC-MS reports suggests the occurrence of 16 compounds. The thrombolytic activity was found significant at test doses in in vivo animal model.
ABOUT AUTHORS
Mr. G Sarvan Kumar (Ph.D), completed his post-graduation in Pharmacology specialization from Dr. M. G. R Medical University, Chennai in 2011. He has total of 10 years of teaching experience. Currently he is working as an Assistant Professor in Vijaya College of Pharmacy, Munaganoor, Hayathnagar, Ranga reddy.
Dr. Malothu Narender, awarded with Ph.D from University College of Pharmaceutical Sciences, Kakatiya University, Warangal in 2016. Currently he is working as an Associate Professor in the department of Pharmaceutical Chemistry at KL College of Pharmacy, KL Deemed to be University, Vijayawada, AP. He is published 20 papers in various International and National Journals.
Dr. D. S. N. B. K. Prasanth is working as an Associate Professor at K L College of Pharmacy, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, AP. He is having 8 years of Teaching and Research experience. He is published 38 papers in various International and National Journals.
Cite this article: Guguloth SK, Malothu N, Prasanth DSNBK. GC-MS Analysis and Thrombolytic Property of Methanolic Leaf Extracts of Terminalia pallida Brandis against Carrageenan Instigated Tail Thrombosis Model in Mice. Pharmacog Res. 2022;14(1):53-60.